|
新辅助治疗对边缘可切除及不可切除III期NSCLC的疗效——怎样欣赏JAMA Onco中的“水文”?!
今天与大家分享一篇分别发表在JAMA Onco上的文献。该研究回顾性分析了美国和意大利两家大型医疗中心的边缘可切除及不可切除的Stage III(T4 and/or N2/N3) NSCLC患者新辅助治疗前后的疗效。作者是怎样基于100多例中位随访两年的回顾性多中心数据,发在JAMA Onco上,一起来看看。
本 文 约3785字 多图预警
认真阅读 需 要 5-10 min
[size=1.6]Neoadjuvant PD-1 and PD-L1 Blockade With Chemotherapy for Borderline Resectable and Unresectable Stage III Non–Small Cell Lung CancerBiagio Ricciuti, MD, PhD; Francesca Fusco, MD; Alissa Cooper, MD; Edoardo Garbo, MD; Federica Pecci, MD; Mihaela Aldea, MD, PhD; Xinan Wang, PhD, MS; Maria Mayoral Penalva, MD; Michelle Ginsberg, MD; Lynette M. Sholl, MD; Mizuki Nishino, MD; Alessandro Di Federico, MD; Narek Shaverdian, MD; Matthew Bott, MD; Valentina Santo, MD; Erino Rendina, MD; Rocco Trisolini, MD; Sara Ramella, MD; Filippo Gallina, MD; Enrico Melis, MD; Simonetta Buglioni, PhD; Gabriele Minuti, MD; Lorenza Landi, MD; Paula A. Ugalde Figueroa, MD; Alice T. Shaw, MD; Jamie Chaft, MD; Mark M. Awad, MD, PhD; Federico Cappuzzo, MD JAMA Oncology May 22 2025
 Importance
Importance Patients with borderline resectable or unresectable stage III non–small cell lung cancer (NSCLC) with T4 and/or N2-N3 involvement face limited treatment options and poor outcomes. Neoadjuvant chemoimmunotherapy has shown promise in improving resectability and pathological responses. 重要性:T4 和/或 N2-N3 受累的临界可切除或不可切除的 III 期非小细胞肺癌 (NSCLC) 患者面临有限的治疗选择和不良结局。新辅助化学免疫疗法在改善可切除性和病理反应方面显示出前景。
Objective To evaluate the efficacy of neoadjuvant programmed cell death 1 protein (PD-1) or programmed cell death 1 ligand 1 (PD-L1) blockade combined with chemotherapy in enhancing surgical outcomes and pathological responses in patients with T4 and/or N2-N3 stage III NSCLC. 目的:评价新辅助程序性细胞死亡 1 蛋白 (PD-1) 或程序性细胞死亡 1 配体 1 (PD-L1) 阻断联合化疗改善 T4 和/或 N2-N3 III 期 NSCLC 患者手术结局和病理反应的疗效。
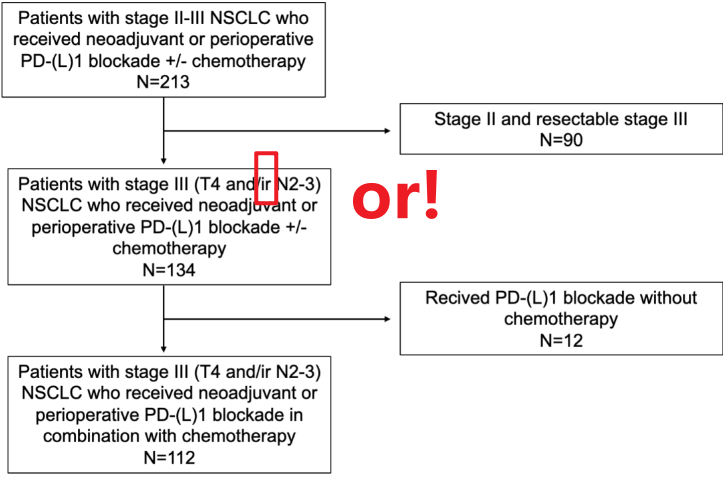
Design, Setting, and Participants This multicenter cohort study analyzed data from patients treated between February 2018 and January 2024 with neoadjuvant PD-1/PD-L1 inhibitors plus chemotherapy at academic and tertiary care centers across the US and Italy. Pathological and survival outcomes were assessed. Patients with stage III NSCLC and T4 and/or N2-N3 involvement were included. Data were collected from February 2018 to January 2024. 设计、设置和参与者 这项多中心队列研究分析了 2018 年 2 月至 2024 年 1 月期间在美国和意大利的学术和三级医疗中心接受新辅助 PD-1/PD-L1 抑制剂联合化疗的患者的数据。评估病理和生存结局。纳入 III 期 NSCLC 和 T4 和/或 N2-N3 受累患者。数据收集时间为 2018 年 2 月至 2024 年 1 月。
Exposures Neoadjuvant PD-1/PD-L1 blockade combined with platinum-based chemotherapy. 暴露 新辅助 PD-1/PD-L1 抑制剂联合铂类化疗。Main Outcomes and Measures Pathological complete response (pCR), major pathological response, surgical resectability, and event-free survival (EFS). 主要结局和测量 病理完全缓解 (pCR) 、主要病理反应、手术可切除性和无事件生存期 (EFS)。 Results Of 112 patients, 58 (51.8%) were female, and the median (range) age was 66 (41-84) years. A total of 84(75.0%) underwent surgical resection, achieving a pCR rate of 29.0% (24 of 83 with available final pathology) and a major pathological response rate of 42.2% (35 of 83). Patients with both PD-L1 expression of 50% or more and high tumor mutational burden achieved the highest pCR rate (4 of 9 [44.4%];

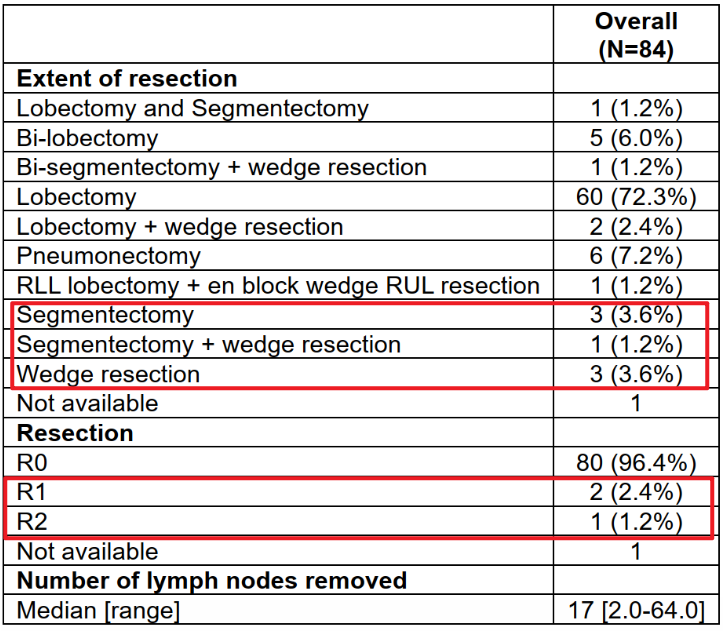
2.上细节(区分事实,畅谈观点): 首先是这篇JAMA Onco在回顾性分析了多中心的新辅助化免患者,重点关注T4 and/or N2/N3的Stage III期患者(这类患者被认为具有较高的肿瘤负荷,常作为临床诊疗中的疑难病例)。与多数研究不同的是该研究声称是一项队列研究(确实样本量也不大),但却纳入了较高比例的腺癌患者,反倒是鳞癌患者不到30%,虽说流程图中排除了一些患者,但这种与多数新辅助研究不一致的鳞癌和腺癌比例让该队列的人群覆盖范围值得怀疑。 其次主要结果四平八稳,看似花哨的图表传递的有效信息极为有限。文章结果呈现上偏离了所声称的针对T4/N2/N3 StageIII NSCLC亚组的关注,反倒是呈现了许多手术队列的常规结果,缺乏对未达到手术切除患者的转化性分析与对比。 最后对于接受手术的N3均转化为ypN0,数据上却缺乏对比分析和详细的数据呈现。对于N3患者没有明确淋巴结清扫范围。最搞笑的是对于手术患者的切除术,肺段和楔形居然有被认为是R0?数据都对不上,以及各种笔误。此外没有从肿瘤学角度出发合理的划分治疗亚组进行探索性分析。有兴趣的兄弟写篇letter吧。。。实在是辣眼睛


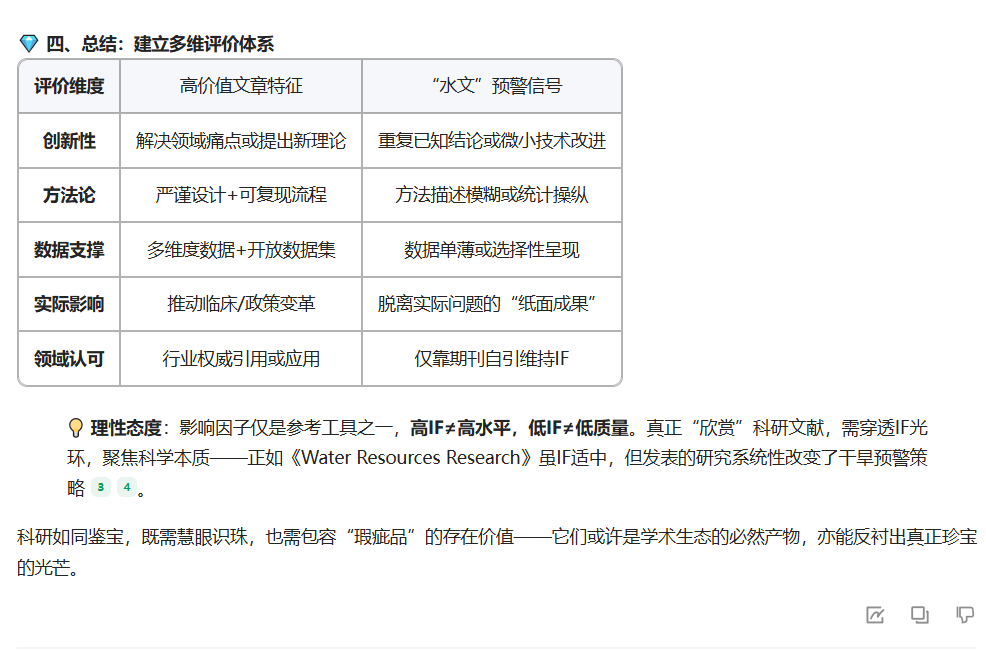
3. 怎样欣赏水文呢?人工智能回答的最后一句颇为亮眼:科研如同鉴宝,既需慧眼识珠,也需包容“瑕疵品”的存在价值——它们或许是学术生态的必然产物,亦能反衬出真正珍宝的光芒。


目录 1. INTRODUCTION 2. Methods 2.1 Patient Population 2.2 Study Design and End Points 2.3 PD-1/PD-L1 Immunohistochemistry 2.4 Next-Generation Sequencing 2.5 Clinical Outcomes Assessment 2.6 Statistical Analysis3.Result 3.1 Patient characteristics (eTables 1 to 3) 3.2 Pathological Outcomes With Neoadjuvant PD-1/PD-L1 Blockade in Combination With Chemotherapy (eFigure 3)(eTable 4-5) (Figure 1)(eFigure 4) (eTable 6)(eFigure 5) 3.3 Impact of PD-L1 Expression, TMB, and Covariant Status on pCR (eFigure 6)(Figure 2B-D) (eFigure 6 B-C)(eFigure 7 -8)(Figure 3A) 3.4 EFS and OS After Neoadjuvant Chemoimmunotherapy (Figure 3B-C)(eFigure 9-12)4. CONCLUSION
—
图表汇总—
2. Methods eFigure 1. STROBE Flowchart
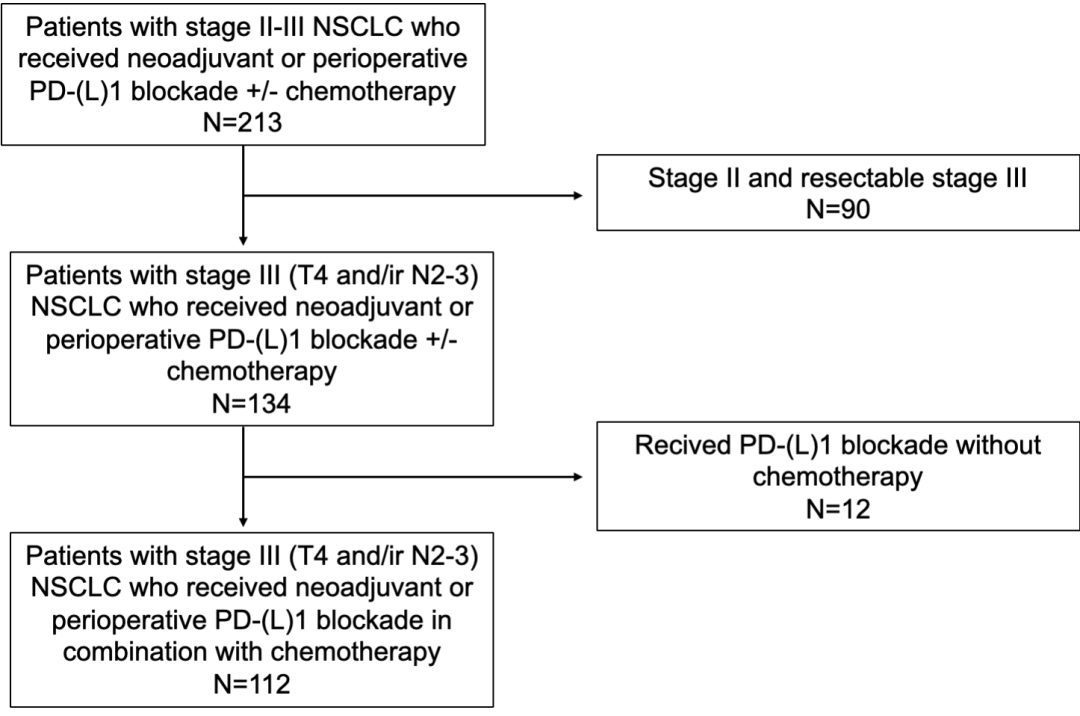
eFigure 2. REMARK Flowchart

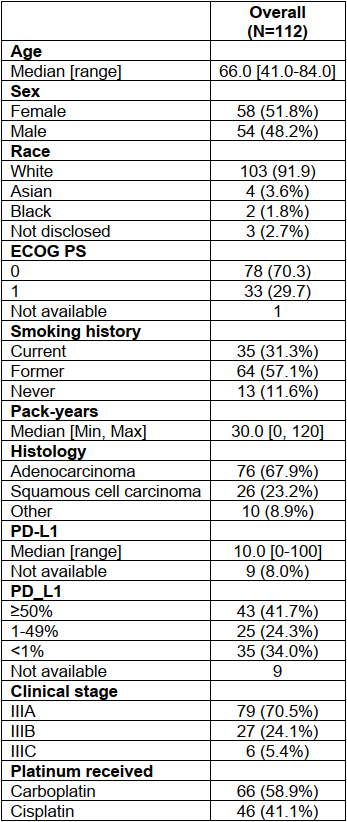
3.1 Patient characteristicseTables 1 Baseline Clinicopathologic Characteristics of Patients With Stage III (T4 and/or N2-3) Enrolled in This Study
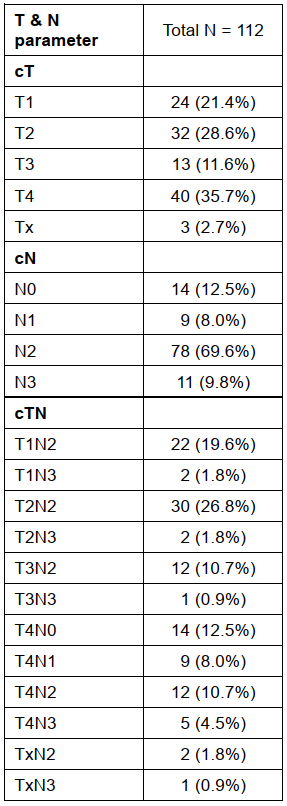
eTables 2 Summary of Baseline T and N Parameter (AJCC eighth edition) Among the 112 Patients Who Received Neoadjuvant Chemoimmunotherapy
eTables 3 Detailed Annotation of the T Parameter in Our Study Cohort
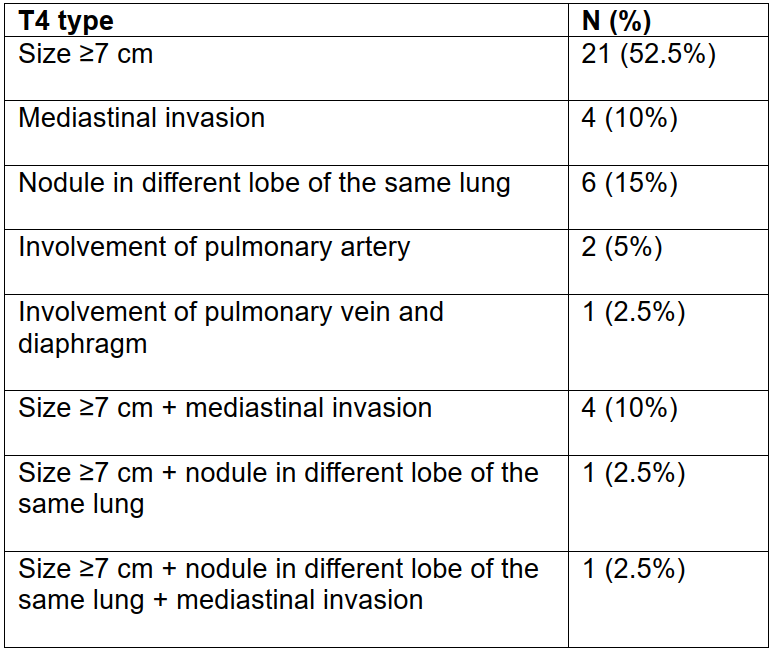
3.2 Pathological Outcomes With Neoadjuvant PD-1/PD-L1 Blockade in Combination With Chemotherapy
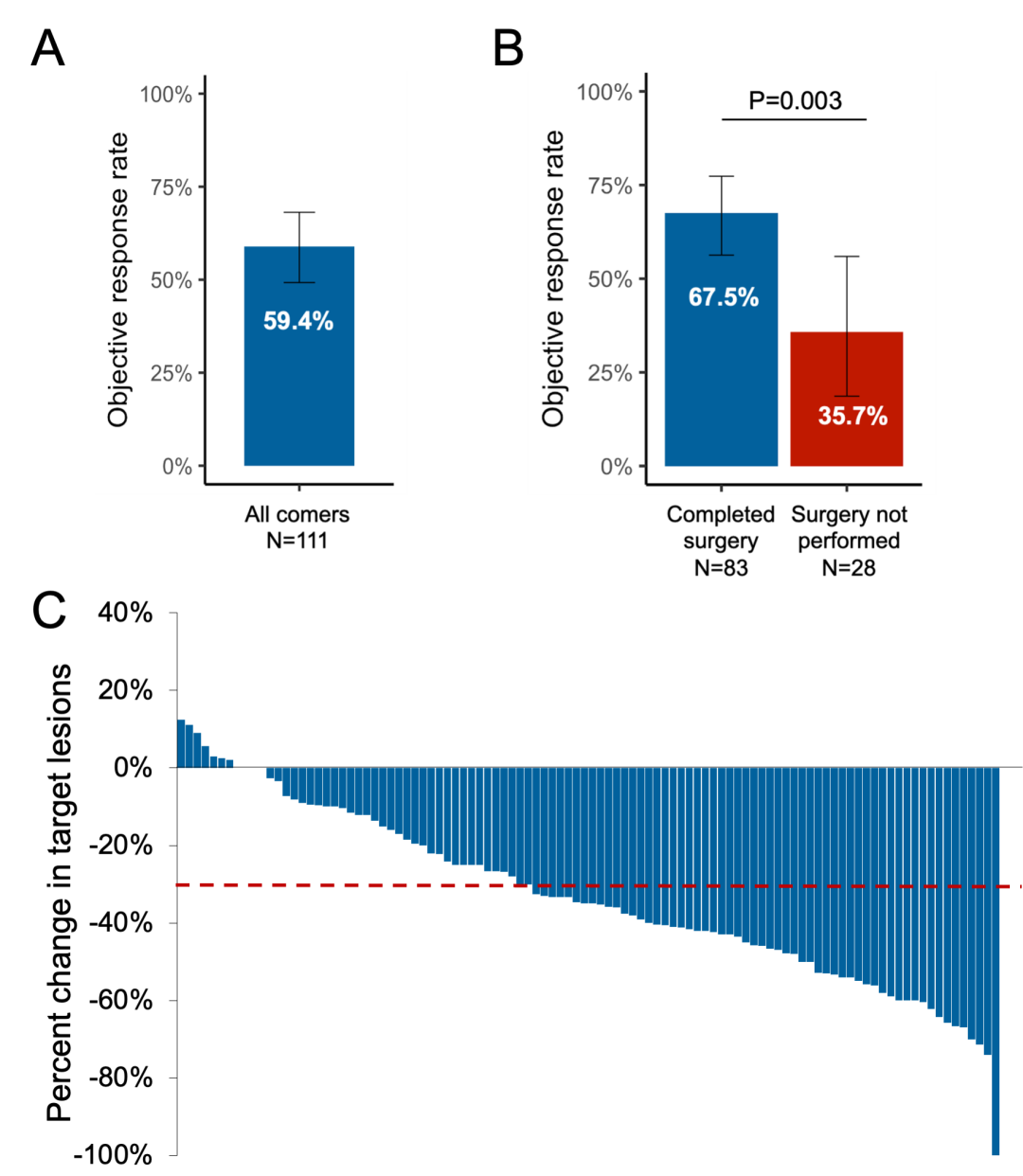
eFigure 3 ORR to Chemoimmunotherapy in the Intention-to-Treat Population and in Patients Who Completed Surgery vs Those Who Did Not Complete Surgery and Percentage Change in Target Lesions After Neoadjuvant Chemoimmunotherapy
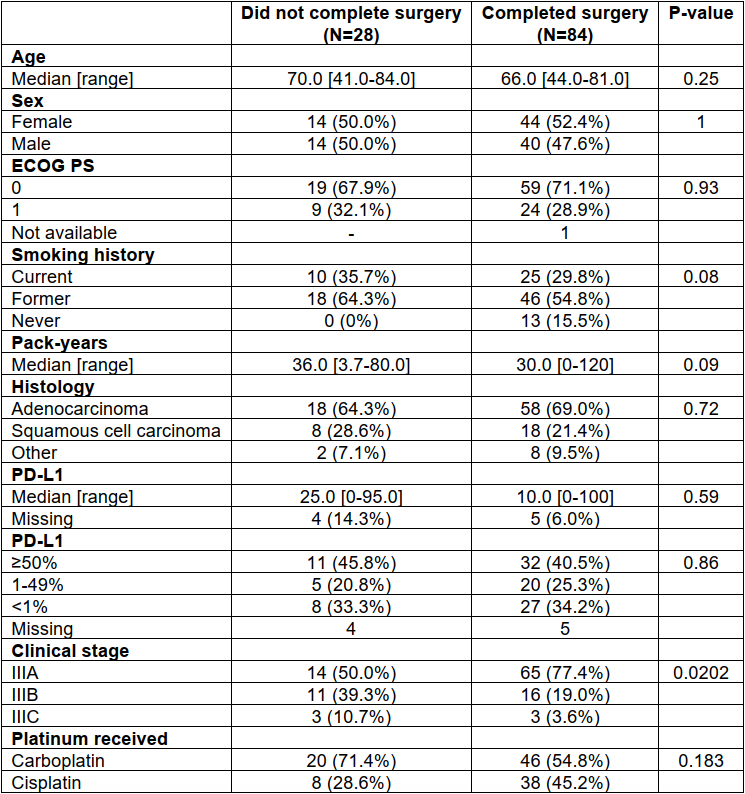
eTable 4 Baseline Clinicopathologic Characteristics of Patients With Stage III (T4 and/or N2-3) Who Did Not Complete Surgery vs Those Did Complete Surgery After Neoadjuvant Chemoimmunotherapy
eTable 5 Extend of Surgical Resection, Residual Tumor, and Number of Lymph Nodes Removed Among 84 Patients Who Underwent Neoadjuvant Chemoimmunotherapy Followed by Surgery
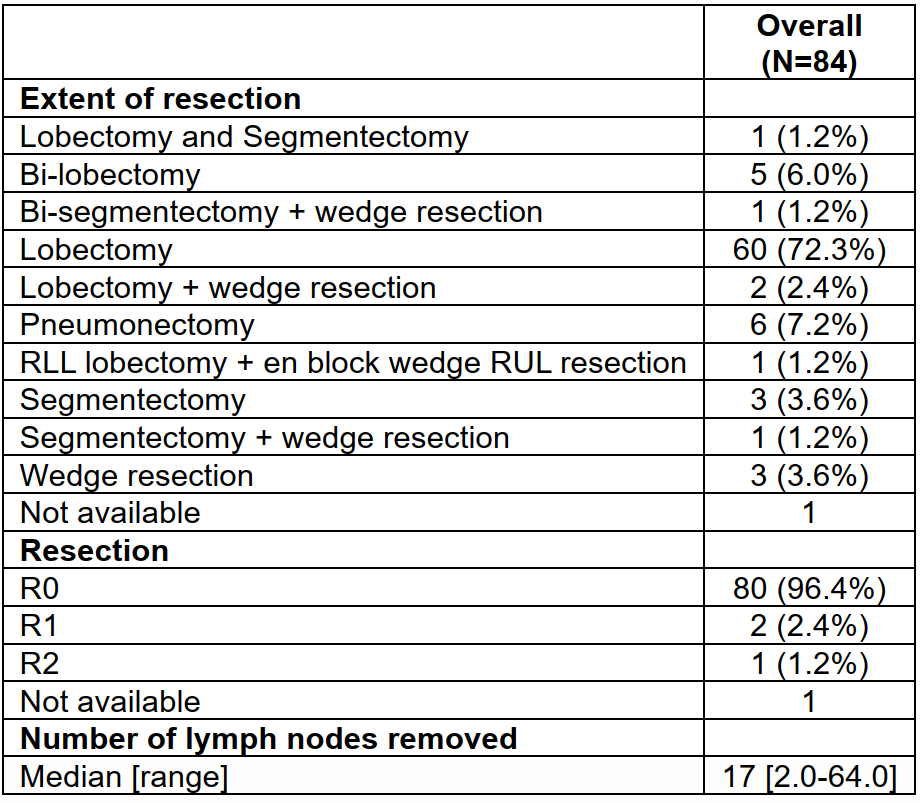
Figure 1 Pathologic Response to Neoadjuvant Chemoimmunotherapy in Patients With Borderline Resectable and Unresectable Stage III Non–Small Cell Lung Cancer
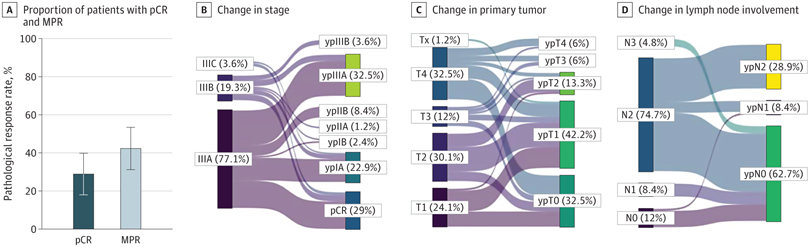
A, Proportion of patients with pathological complete response (pCR) and major pathological response (MPR) among patients who underwent neoadjuvant chemoimmunotherapy followed by surgical resection. B-D, Sankey diagrams showing changes in stage (B), primary tumor (C), and lymph node involvement (D) before and after neoadjuvant chemoimmunotherapy followed by surgery. Error bars indicate 95% CIs.
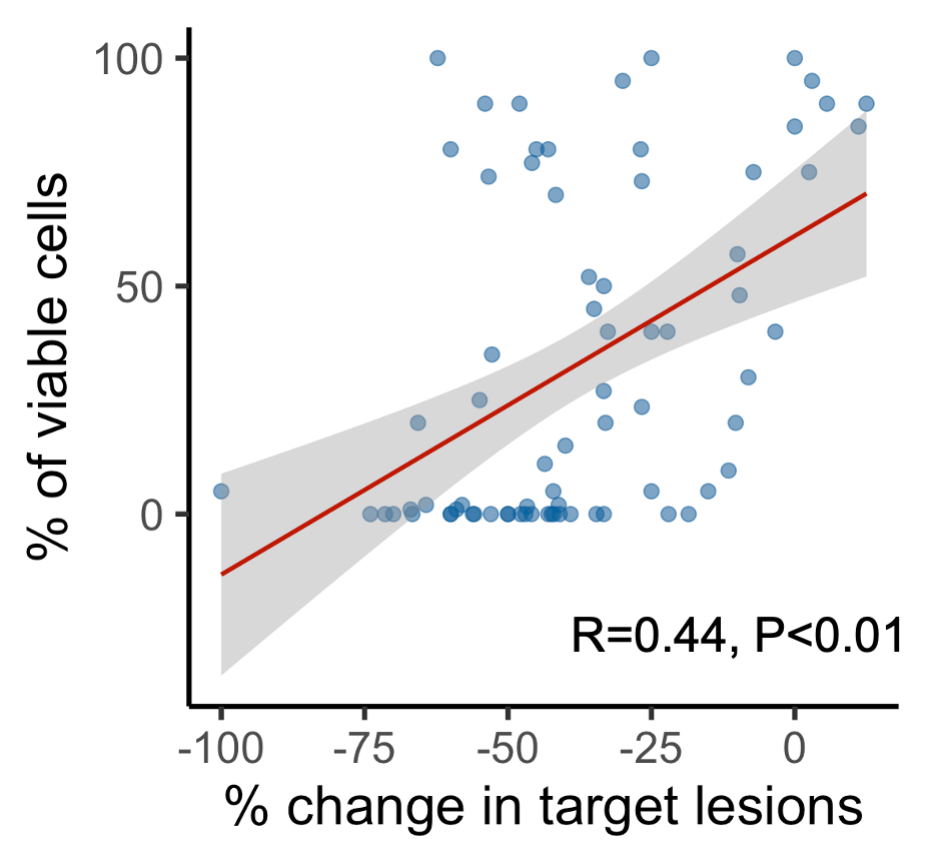
eFigure 4 Correlation Between Depth of Radiologic Response After Neoadjuvant Chemoimmunotherapy and Percentage of Viable Tumor Cells After Surgery
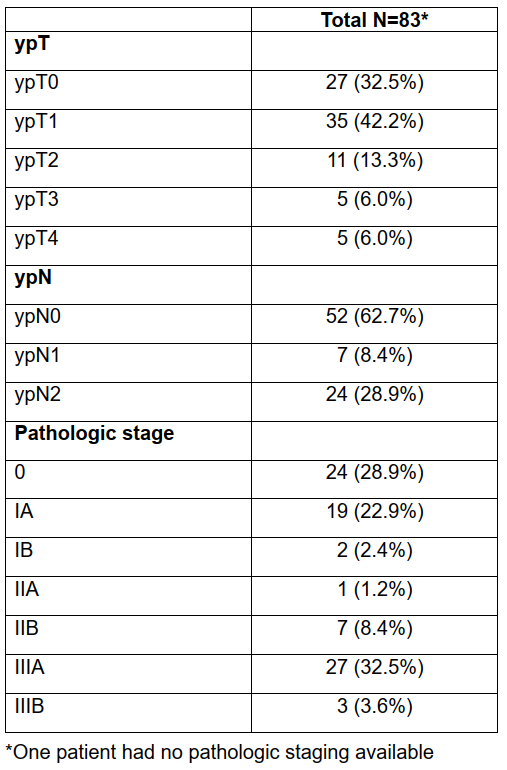
eTable 6 Pathology Staging Among 84 Patients Who Underwent Neoadjuvant Chemoimmunotherapy and Successfully Completed Surgery
eFigure 5 Association of pCR Rate by Subgroup
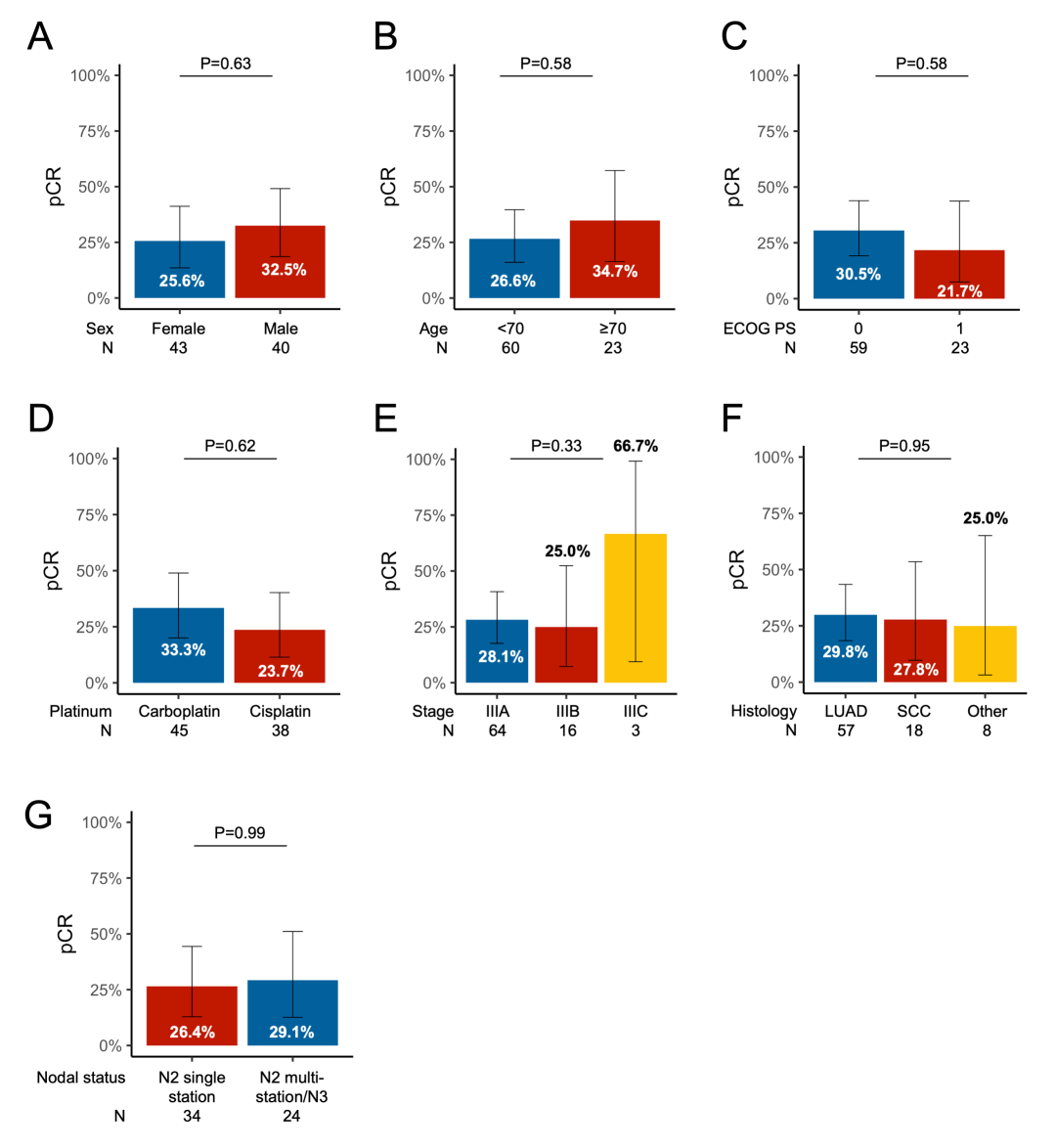 Association of pCR rate with (A) sex, (B) age, (C) ECOG PS, (D) type of platinum received, (E) stage, (F) histology, and nodal involvement.
Association of pCR rate with (A) sex, (B) age, (C) ECOG PS, (D) type of platinum received, (E) stage, (F) histology, and nodal involvement.
3.3 Impact of PD-L1 Expression, TMB, and Covariant Status on pCReFigure 6 Difference in Median PD-L1 and TMB Between Patients With and Without pCR and pCR Rate Among Patients With High vs Low TMB
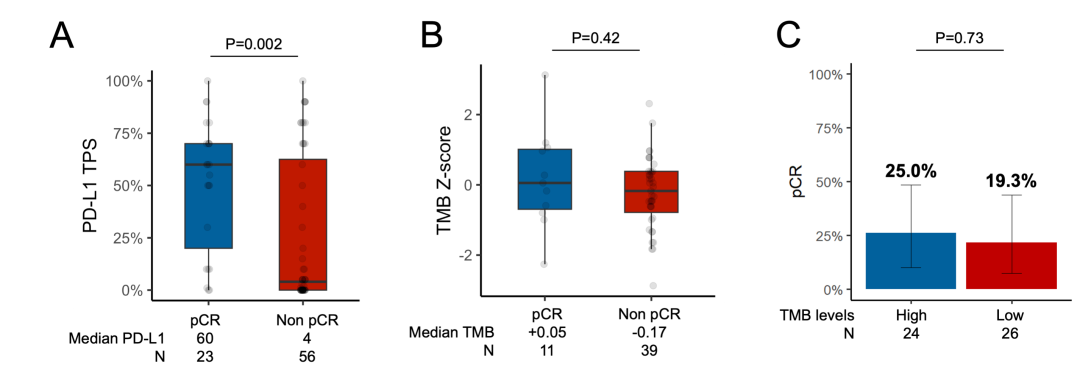
(A) Difference in median PD-L1 between patients with and without pCR. Tumor mutational burden in patients with versus without pCR (B). pCR rate among patients with high (≥50th percentile of harmonized TMB) versus low (<50th percentile) TMB (C). See Supplementary Figure 2 for REMARK flow chart.
Figure 2 Clinicopathologic and Genomic Correlates of Pathological Complete Response (pCR) in Borderline Resectable and Unresectable Stage III Non–Small Cell Lung Cancer
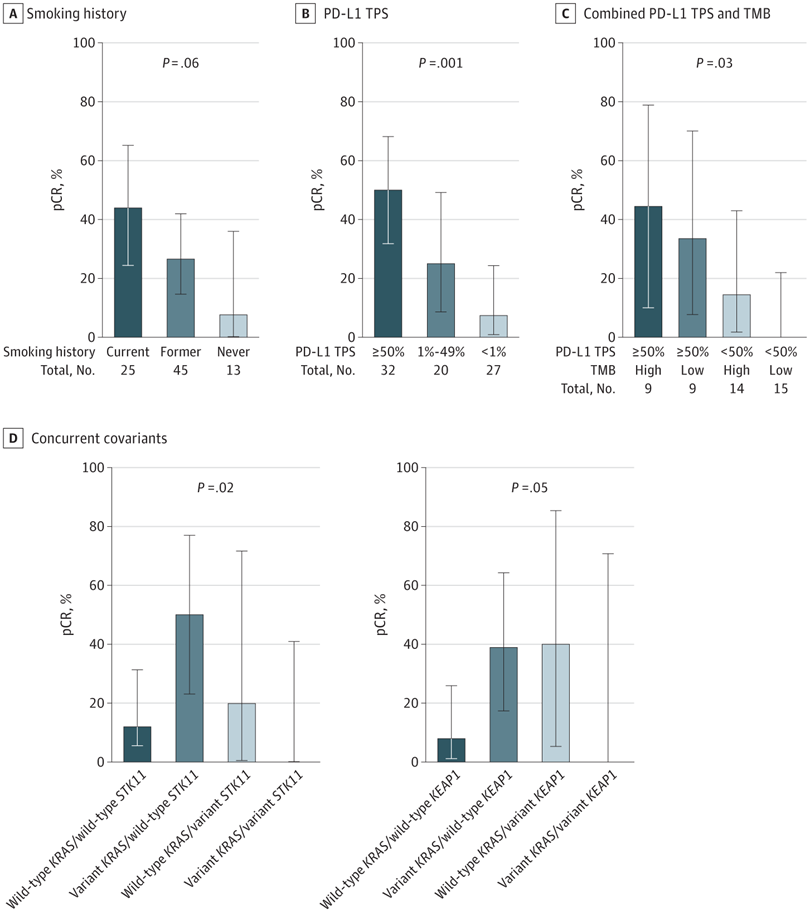 Clinical and pathological correlates of pCR with smoking history (A) and clinically relevant cutoffs of programmed cell death 1 ligand 1 (PD-L1) tumor proportion score (TPS) (B) as well as genomic correlates of pCR with combined PD-L1 and tumor mutational burden (TMB) (C) and concurrent KRAS/STK11 and KRAS/KEAP1 (D). Error bars indicate 95% CIs.
Clinical and pathological correlates of pCR with smoking history (A) and clinically relevant cutoffs of programmed cell death 1 ligand 1 (PD-L1) tumor proportion score (TPS) (B) as well as genomic correlates of pCR with combined PD-L1 and tumor mutational burden (TMB) (C) and concurrent KRAS/STK11 and KRAS/KEAP1 (D). Error bars indicate 95% CIs.
eFigure 6 Difference in Median PD-L1 and TMB Between Patients With and Without pCR and pCR Rate Among Patients With High vs Low TMB
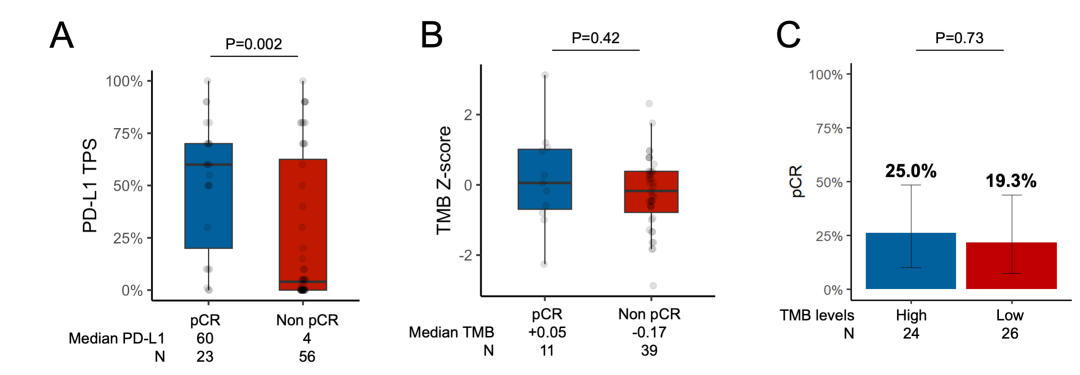
(A) Difference in median PD-L1 between patients with and without pCR. Tumor mutational burden in patients with versus without pCR (B). pCR rate among patients with high (≥50th percentile of harmonized TMB) versus low (<50th percentile) TMB (C). See Supplementary Figure 2 for REMARK flow chart.
eFigure 7 pCR Rate by Variant Status
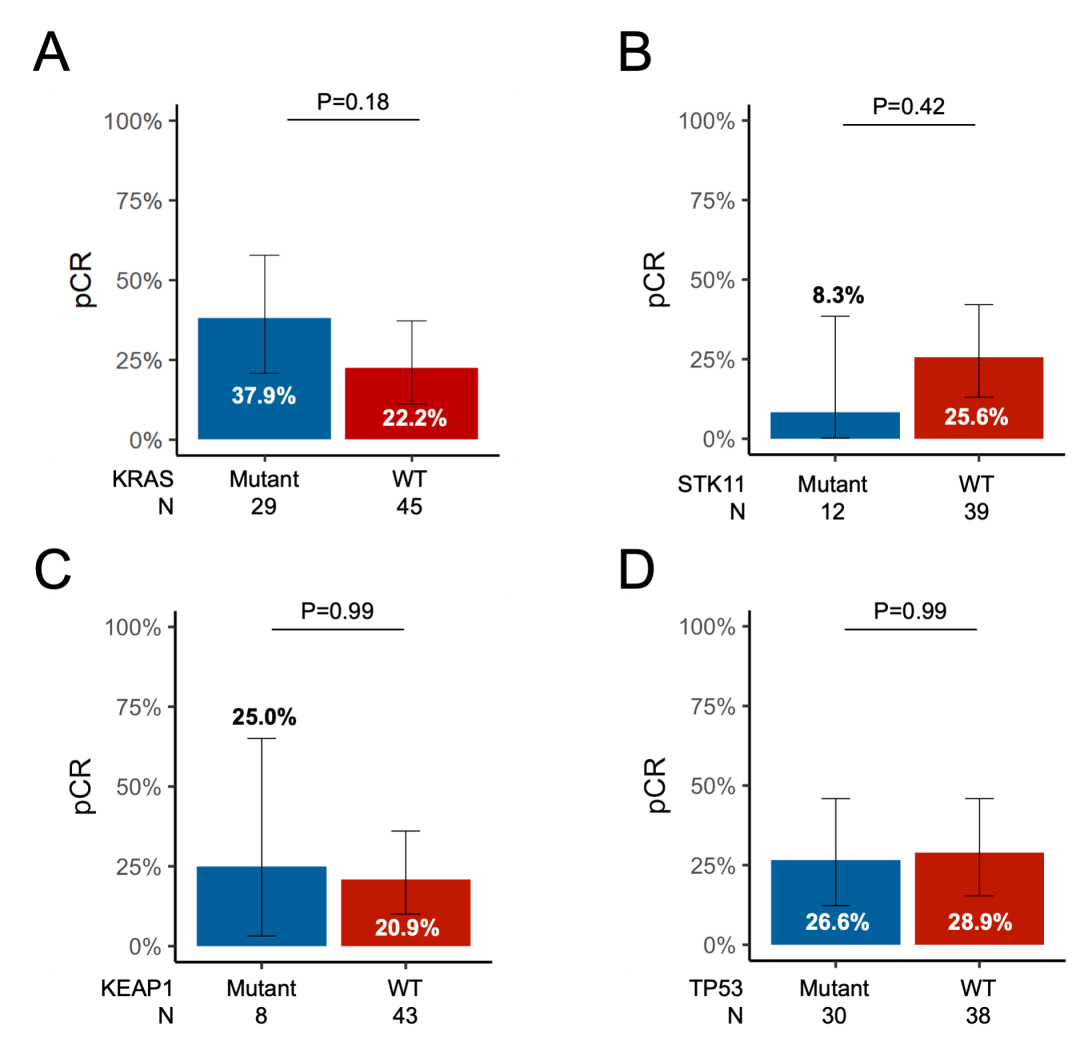
pCR rate according to (A) KRAS, (B) STK11, (C) KEAP1, and (D) TP53 mutation status. See Supplementary Figure 2 for REMARK flow chart.
eFigure 8 pCR Rate by KRAS/TP53 Covariant Status
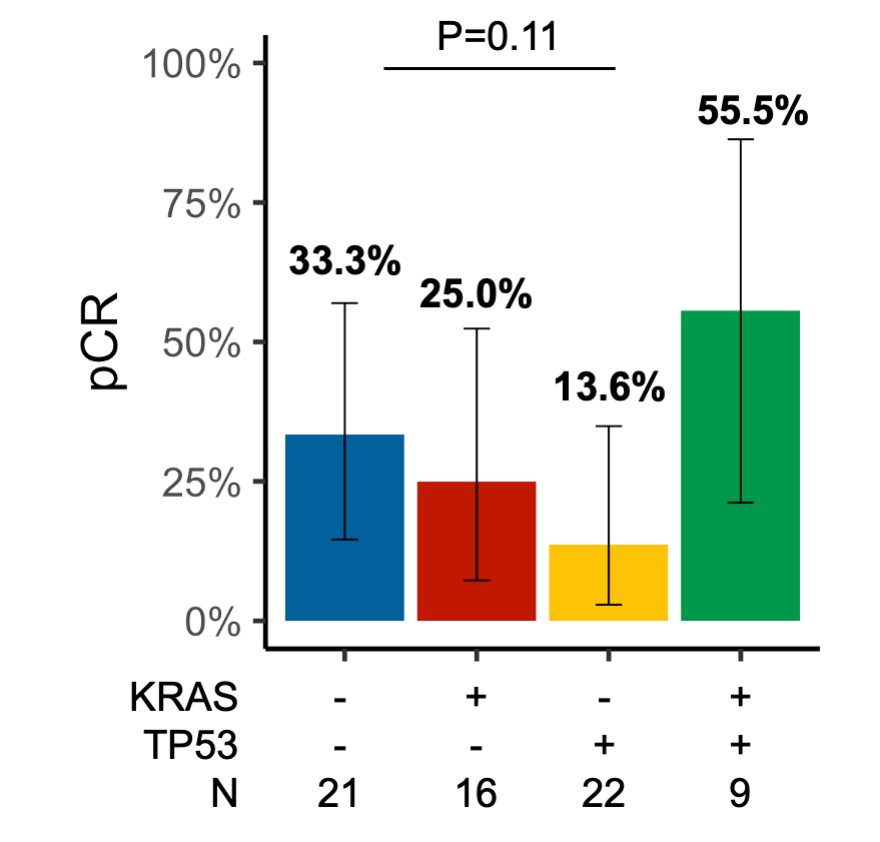
pCR rate according to (A) KRAS/TP53 co-mutation status. See Supplementary Figure 2 for REMARK flow chart.
Figure 3A Overview of the Clinicopathologic and Genomic Determinants of Complete Pathologic Response (pCR) and Major Pathological Response (MPR) and Survival Outcomes With Neoadjuvant Chemoimmunotherapy in Borderline Resectable and Unresectbale Stage III Non–Small Cell Lung Cancer
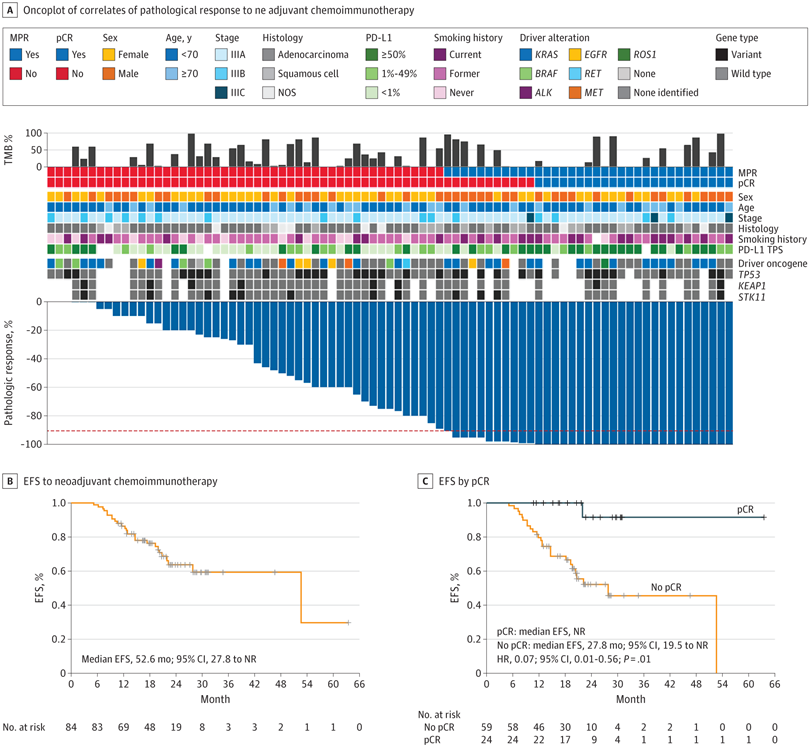 A, Oncoplot showing the clinicopathological and genomic correlates of pathological response to neoadjuvant chemoimmunotherapy.
A, Oncoplot showing the clinicopathological and genomic correlates of pathological response to neoadjuvant chemoimmunotherapy.
3.4 EFS and OS After Neoadjuvant Chemoimmunotherapy Figure 3 B, Event-free survival (EFS) to neoadjuvant chemoimmunotherapy among patients with T4 and/or N2-3 non–small cell lung cancer who completed surgical resection. C, EFS according to pCR. HR indicates hazard ratio; NOS, not otherwise specified; NR, not reached; PD-L1, programmed cell death 1 ligand 1; TMB, tumor mutational burden; TPS, tumor proportion score.
eFigure 9 Overall Survival Among the 84 Patients Who Received Neoadjuvant Chemoimmunotherapy and Underwent Surgery and Event-Free Survival Among Patients With vs Without MPR
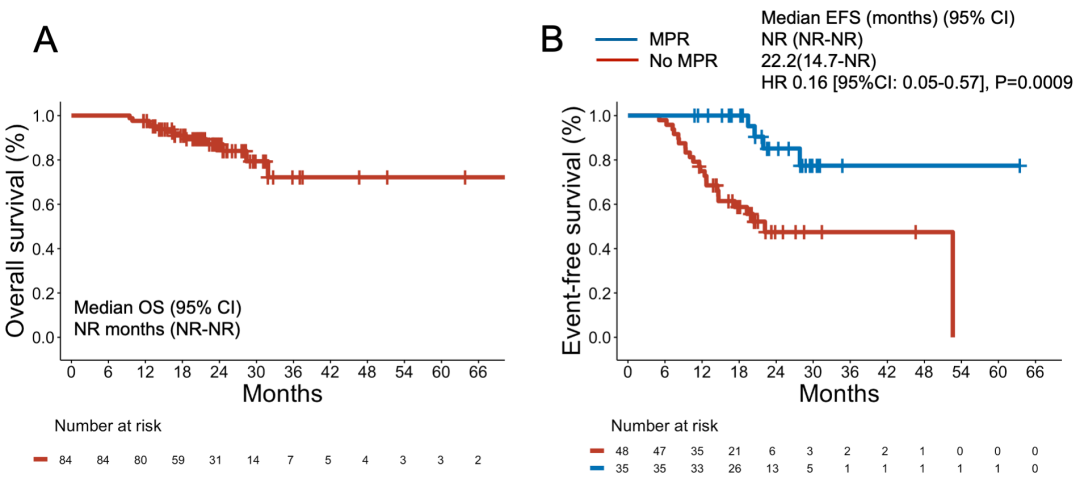
(A) Overall survival among the 84 patients who received neoadjuvant chemo-immunotherapy and underwent surgery. (B) Event-free survival among patients with versus without MPR.
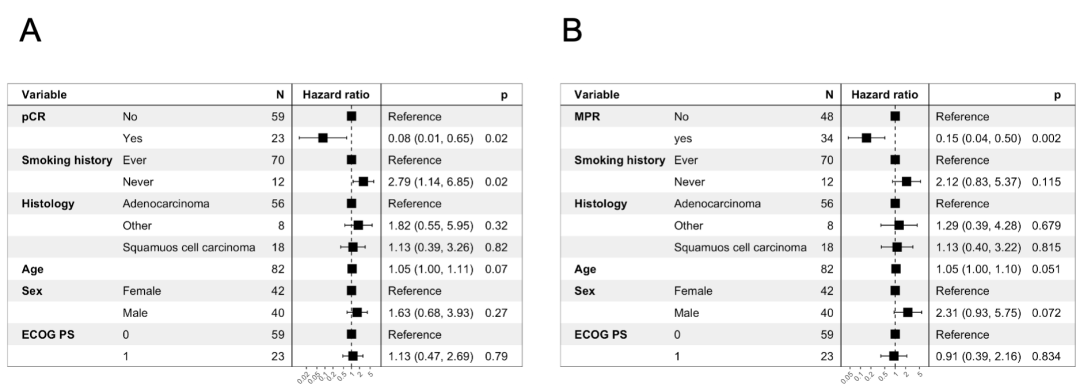
eFigure 10 Multivariable Cox Regression Models for Event-Free Survival in Patients With vs Without pCR and MPR
eFigure 11 Overall Survival After Neoadjuvant Chemoimmunotherapy Among Patients With vs Without pCR or MPR
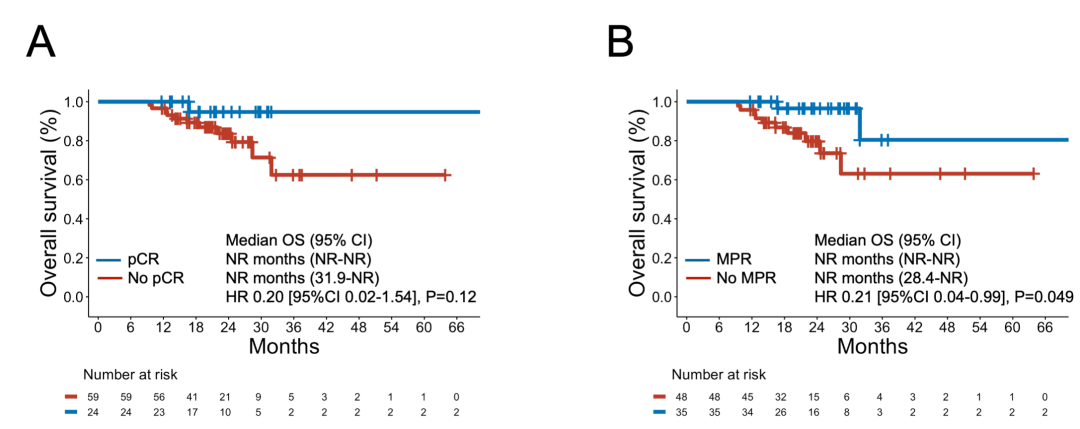
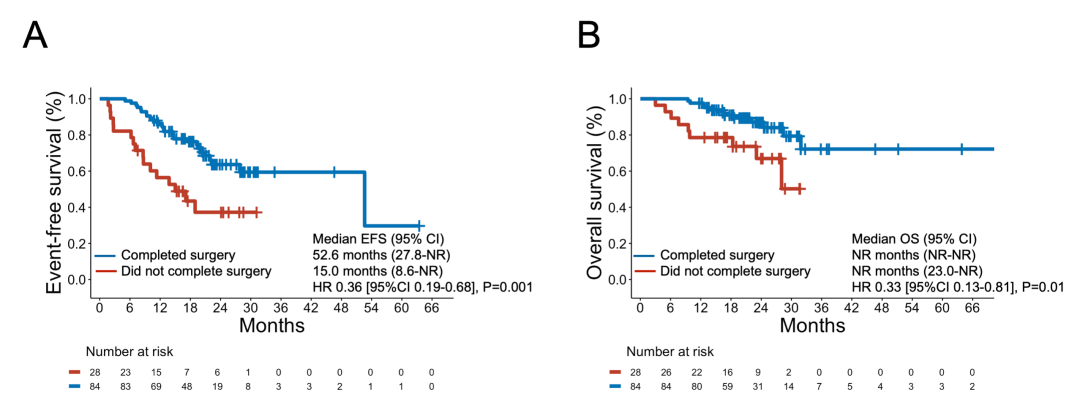
eFigure 12 Event-Free Survival and Overall Survival Among Patients Who Completed Surgery and in Those Who Did Not Complete Surgery After Neoadjuvant Chemoimmunotherapy
巳巳如意~!
|